Miscibility of Hydrocarbon Systems
In petroleum engineering, we consider two types of miscibility: first-contact miscibility (FCM) and multi-contact miscibility (MCM). Miscibility is an important enhanced oil recovery (EOR) process to reduce the magnitude of residual oil saturation (towards zero) in the gas-swept pores. These two types of miscibility are discussed below.
The minimum conditions of pressure and injected gas enrichment (e.g. \(C_2\)-\(C_5\)) to develop miscibility are important design criteria to minimize costs – namely, minimum miscibility pressure (MMP) for a specific injection gas, and the less-common minimum miscibility enrichment (MME) for a target injection/reservoir pressure maintenance.
Three primary mechanisms can lead to a miscible displacement front where residual oil saturation is driven towards zero in the gas-swept pores. The most prevalent miscible gas process is called the condensing/vaporizing (C/V) mechanism, responsible for developed miscibility when injecting hydrocarbon-rich separator gases and \(CO_2\). For leaner injection gases consisting mainly of \(N_2\), methane and ethane, the mechanism of developed miscibility is usually the vaporizing gas drive (VGD) process. The least common miscibility process, relevant only to huff-n-puff EOR processes in tight unconventionals, is first-contact (FC) miscibility, where the gas being injected and the in-situ reservoir fluids are directly miscible at reservoir conditions in the pores where mixing occurs.
Minimum Miscibility Pressure
For a given temperature, injection gas composition, and reservoir fluid composition, the minimum miscibility pressure (MMP) is the lowest pressure at which miscibility can be achieved for a specific miscibility process, i.e. \(MMP_{C/V}\) by condensing/vaporizing gas drive, \(MMP_{VGD}\) by vaporizing gas drive, and \(MMP_{FC}\) by first contact.
First Contact Miscibility
At a given pressure and temperature, first-contact (FC) miscibility refers to the process in which two fluids mix in all proportions – at any concentration of either fluid – such that the resulting mixture remains a single phase. This is the formal, text-book definition of “miscibility”.
In petroleum systems, we use the term first-contact minimum miscibility pressure to define the pressure at which the injection gas and reservoir fluid mix on first contact, in any proportion, forming a single phase. \(MMP_{FC}\) is obtained from a swell test, defined as the maximum pressure on a pressure-composition (p-x) diagram plotting saturation pressure vs. mol-% injection gas. A p-x diagram showing \(MMP_{FC}\) is shown in Figure 1.
At p\(\geq MMP_{FC}\), all mixtures of injected gas and reservoir fluid are single-phase, and are therefore the system is first-contact miscible.
At p\(<MMP_{FC}\), some mixtures of injected gas and reservoir fluid form two equilibrium phases, and therefore the system is not first-contact miscible.
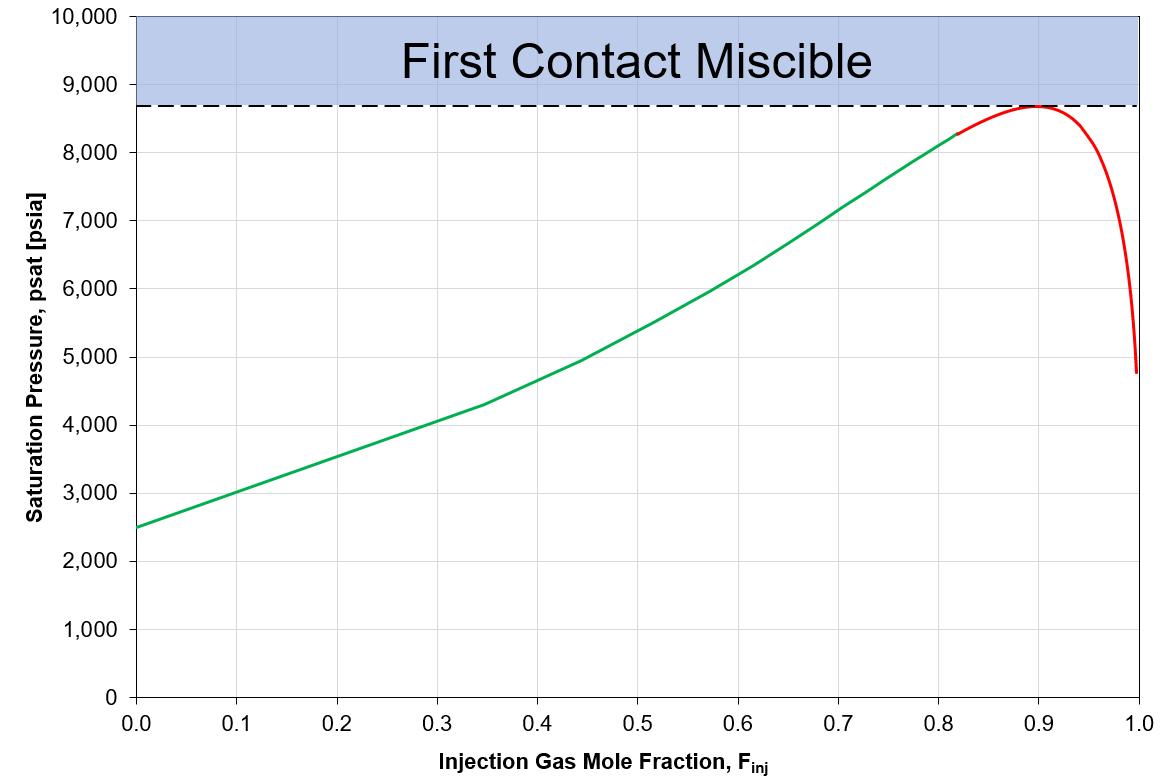
Figure 1: Example of swell test data and indicated MMP by first contact.
Multi-Contact Miscibility
In a conventional displacement process, miscibility between a reservoir fluid and an injection gas develops through a dynamic process of mixing, with complex component exchange controlled by phase equilibria (K-values) and local compositional variations along the path of displacement. In such a process, the injection gas and reservoir fluid are said to be multi-contact miscible (MCM). In reality, multi-contact miscibility is a complex, dynamic process that cannot always be simulated or created in a PVT cell. The slimtube test should capture the multi-contact miscibility process and identify the achievement of miscibililty (or not), without providing details of the in-situ compositional changes between the point of injection and the point of production.
Vaporizing and Condensing Gas Drive
whitson comment
Note from Markus Hays Nielsen: The condensing gas drive mechanism does not appear in real hydrocarbon systems.
Before 1986, it was believed that developed, multi-contact miscibility followed one of two paths: (1) vaporizing gas drive (VGD), developed and maintained at the gas front; or (2) condensing gas drive (CGD), initiated at the point of injection behind the gas front.
In a VGD process, the injection gas becomes continuously enriched in \(C_{3+}\) components by repeated contacts with the original reservoir oil. Miscibility develops at the gas front between the enriched frontal gas and the original reservoir oil. Zero gas saturation exists ahead of the miscible front in a VGD process, a signature of the VGD process, and the reason why VGD miscibility will always develop above the reservoir oil’s bubblepoint pressure.
In a theorized CGD process, the injection gas contains light intermediates (e.g. (\(C_{2}\)-\(C_{5}\)) that partition into the reservoir oil at the point of injection. CGD theory assumes that the reservoir oil’s enrichment with the light intermediates will continue until the altered oil becomes first-contact miscible with the injected gas. In reality, such miscibility will seldom (if ever) develop. Zick showed that for each contact of fresh injection gas with the oil at the point of injection, net enrichment slows because the reservoir oil becomes fully saturated with these light intermediates. Zick points out that each and every new contact of fresh injection gas with the changing oil will lead to continuous vaporization of heavier components from the reservoir oil, components that are not found in the injection gas (e.g. \(C_{6+}\)). The negative effect of \(C_{6+}\) vaporization will never be sufficiently compensated by the early-contact enrichment with light intermediates, and the oil near the point of injection does not develop miscibility with injected gas (as originally proposed in the literature for oils, but which is only valid for ternary systems)1\(^,\)2.
Condensing-Vaporizing Gas Drive
In 1986, Zick was the first to show that a combined condensing and vaporizing (C/V) mechanism describes the actual development of minimum miscibility for most naturally occurring petroleum systems. He showed that the point of miscibility, i.e. ~100% recovery efficiency in a dispersion-free 1D displacement, is somewhere between the point of injection and the gas front, as shown in Figure 2.
Zick (1986) also found that the MMP predicted by the C/V mechanism could be significantly lower than the one predicted by the VGD mechanism; findings that have later been verified by many 3\(^,\)4\(^,\)5.
In the C/V process, a near-miscible front develops inside the reservoir, and likely within the first few feet away from the point of injection. The near-critical front that develops is flanked on the upstream side with “severe” (near-critical) vaporization, where oil saturation approaches zero. Severe condensation is found just downstream to the near-critical front, greatly altering the oil that is contacted by near-critical frontal gas that has developed behind the front. C/V miscibility is a complex and dynamic process of fluid mixing and near-critical phase behavior that is independent of rock properties (relative permeability), phase viscosities and gas-oil interfacial tension.
An “inactive” gas saturation may exist or develop ahead of the condensing side of the C/V miscible front. In this inactive zone with non-zero gas saturation, insignificant component exchange between the oil and gas phases exists, as the two phases are in thermodynamic equilibrium in this inactive zone. The existence and extent of this inactive gas-oil saturated zone ahead of the C/V miscible front has no impact on the development and maintenance of miscibility. The inactive zone may disappear completely if the initial oil is highly undersaturated at frontal displacement pressures, and in such cases the condensing near-critical side of the C/V miscible front will require very fine gridding to see its existence and importance to the process.
The C/V miscibility process can develop at pressures below the initial bubblepoint of the original reservoir oil, or in a reservoir that has undergone depletion and exists in a two-phase, gas-oil state prior to and during the EOR process.
Zick showed that the point of miscibility, i.e. near-100% recovery efficiency in a dispersion-free 1D displacement, develops near the point of injection and propagates throughout the displacement path taken in the reservoir. Figure 2 is an example snapshot of the typical C/V mechanism with hour-glass shape when plotting gas and oil densities, K-values of \(C_{1}\) and \(C_{7+}\), or gas-oil IFT; the miscible front is define as the point where the hour glass “size” is at a minimum.
The C/V miscibility process also develops when injecting \(CO_{2}\). We are not aware of any examples where pure \(CO_{2}\), or high-content \(CO_{2}\) injection does not result in a C/V process at the MMP. Furthermore, most injection gases using separator gas directly or a lightly-processed separator gas, will usually develop MMP conditions by the C/V process.
Zick discusses that the C/V process can only be studied with a dynamic multi-contact mixing process, e.g. a slimtube (1D displacement with minimal dispersion). He also developed in 1986, without publishing the method, an efficient multi-cell simulation algorithm that provided an accurate MMP estimate with minimal computations. The method made use of quantifying and extrapolating the C/V hour-glass “minimum” size to “zero” at some minimum pressure, the \(MMP_{C/V}\). Zick shared the algorithm strategy with Lynn Orr in 1987, and Curtis Whitson circa 1990. Whitson shared the algorithm (with Zick’s consent) through courses and private communications, including a visit requested by Jean-Noel Jaubert and his PhD advisor Evelyne Neau in 1996, following Whitson’s mention of the method at a lecture he held in Paris.
Publications of multi-cell algorithms mimicking Zick’s original algorithm were published without reference to Zick, despite the authors knowing that Zick had first developed this approach at Arco in the mid-1980s.
Zick also showed that the C/V MMP could be (and often is) significantly lower than the MMP predicted by a purely vaporizing gas drive mechanism. We have mentioned that the C/V MMP can be lower than the original oil bubblepoint, or in a depleted two-phase, gas-oil state prior to and during the displacement process. The VGD MMP must always be at a pressure higher than the bubblepoint pressure of the reservoir oil (or dewpoint of a reservoir gas condensate).
A miscible displacement that develops through the VGD process is usually associated with the injection of a “lean” gas consisting mainly of methane or nitrogen, with only smaller contents of ethanes and heavier hydrocarbons.
All of the observations made by Zick in his pioneering 1986 paper have been verified time and again by similar numerical PVT and 1D simulation studies, and by attempts to prove the C/V mechanism using the method of characteristics. None of these subsequent studies found any of Zick’s original observations to be wrong, or misleading. Instead, they all verified the original discussions, observations and conclusions given by Zick in 1986.
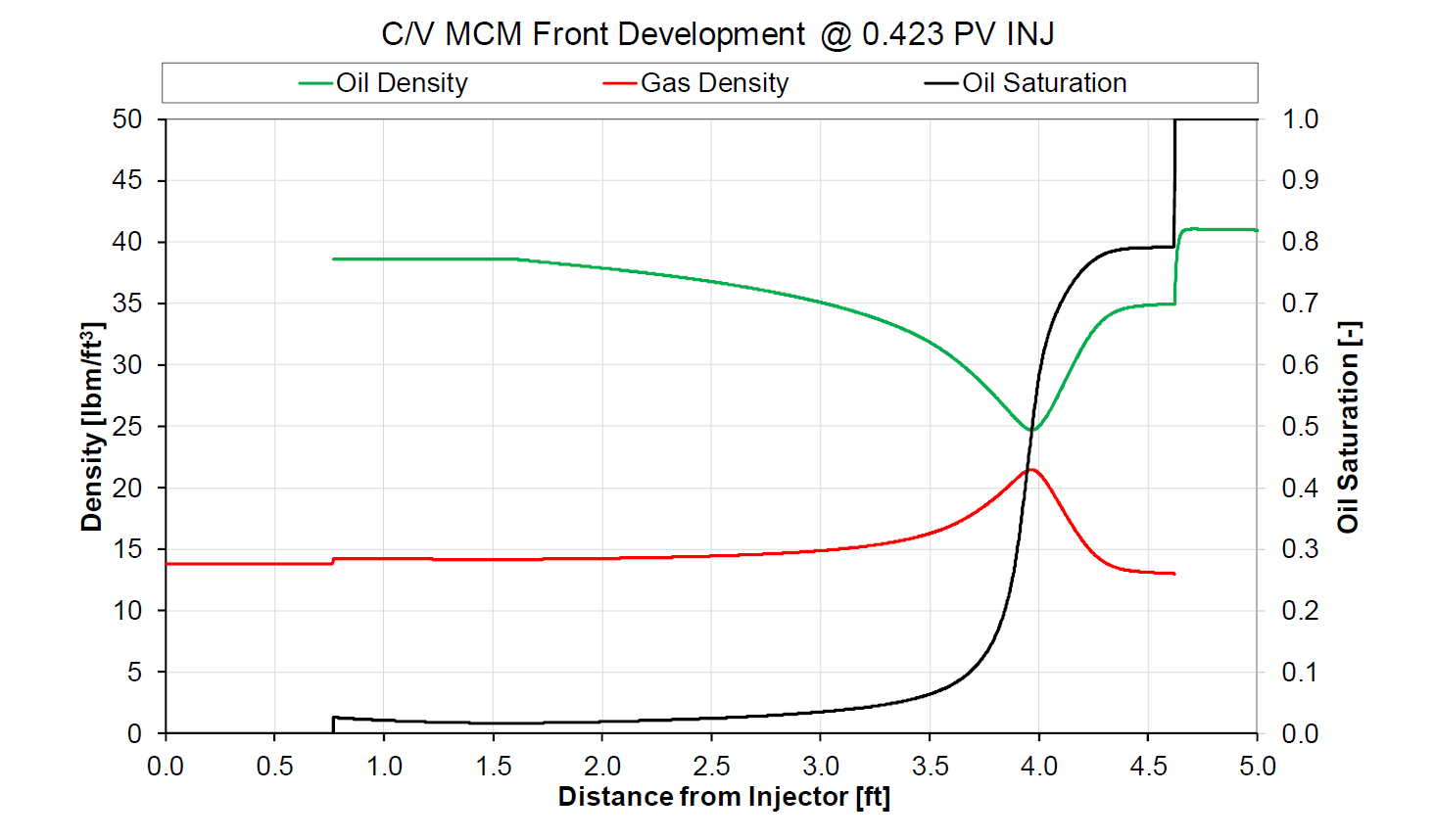
Figure 2: Example of multi-contact miscibility by C/V gas drive.
Calculating the MMP by Multi-Contact
In Zick's 1987 paper, he suggested that a minimum of four components was needed for the C/V mechanism to occur. This prompted further study of the condencing/vaporizing mechanism. The results of this additional research produced two approaches for estimating the MMP by calculation where the first was proposed by Zick called a multiple-mixing-cell method6\(^,\)7 and another was proposed by Orr, Wang, Jessen and others3\(^,\)4\(^,\)8\(^,\)9 based on more complicated tie-line algorithms. The tie-line approaches have proven to have significant issues for certain cases, while the multiple-mixing-cell is found to be stable and reliable.
Multiple-Mixing-Cell Approach
Aaron Zick invented a mixing-cell MMP algorithm in 1986 (basically: using multiple mixing cell contacts, extrapolation of the shortest tie line to an infinite number of contacts, and search for a pressure at which that extrapolated tie line first goes to zero length, also allowing for positive and negative tie line lengths). Zick also uses the same algorithm to determine minimum miscibility enrichment of a solvent at fixed pressure (MME). Zick’s method was made commercially 20 years ago, in 2001 (MMPz), and later in 2005 when marketed as a feature in Zick’s EOS modeling software PhazeComp (www.zicktech.com).
Several authors have plagiarized Zick’s mixing-cell MMP methods in publications. None of these proposed methods proved superior (in terms of accuracy and speed) to Zick’s original method from 1986, or that made commercially available in 2001.
Measuring the MMP by Multi-Contact Experimentally
Obtaining the \(MMP_{MC}\) experimentally is difficult and a variety of these are reported by Zhang et al.7. Three types of experiments are described below.
Slimtube Experiment
The slimtube experiment typically consists of a 40-80 ft coiled tube with a 1/4 inch inner diameter that is packed with sand or glass beads (often in the 100 mesh range).The tube is initially filled with oil at a pressure above the oil’s saturation pressure. Gas is then injected at one end, and the final oil recovery after 1.2 pore-volumes injected (PVI) is noted. Each displacement is done at a specific pressure, making it possible to plot the recovery factor (RF) at 1.2 PVI vs. pressure.
The first attemt at a slimtube experiment was proposed by Yarborough10 in 1970. However, the "standard" procedure was presented by Yelling and Metcalfe11 in 1980.
Rising-Bubble Apparatus
The idea of the rising bubble apparatus (RBA) is to let bubbles of gas rise through a small glass tube (typically rectangular shaped with dimensions 1x5x200 mm) filled with oil at different pressures. The rising bubbles, and their shape, are observed by a camera, and the lowest pressure at which the bubble disappears during rise (i.e. dissolves) is considered the \(MMP_{RBA}\). An important note is that the RBA was designed around the time of Zick's discovery of the C/V mechanism and was therefore not designed to be predict \(MMP_{C/V}\).
The rising-bubble apparatus (RBA) was introduced by Christiansen and Haines12 in 1987 as a fast alternative to the slimtube.
Vanishing Interfacial Tension
The idea of the vanishing interfacial tension (VIT) experiment is to have a transparent cell that is initially charged with the injection gas and reservoir oil (typically with a ~10 vol% of oil). There is a capillary tube at the top of cell used to inject oil droplets into the cell. The pressure is maintained at a specified level by injecting gas into the cell. The initial amount of gas and oil in the cell is allowed to equilibrate for ~1 hour before the first oil droplet is introduced through the capillary tube. The shape of the droplet is photographed, and the IFT13 is estimated based on the Young-Laplace equation based on the physical shape of the droplet. This is done repeatedly at increasing pressures until the droplet disappears. An IFT vs. pressure plot is made, where the points are fit using an appropriate function. The curve is then extrapolated to the pressure that gives zero IFT, and this is deemed the \(MMP_{VIT}\).
The vanishing interfacial tension (VIT) was introduced by Rao14 in 1997 for the same reason as the RBA, to obtain MMP measurements faster than the slimtube.
-
A. Zick. A combined condensing/vaporizing mechanism in the displacement of oil by enriched gases. In SPE annual technical conference and exhibition, paper SPE 15493. Society of Petroleum Engineers, 1986. doi:10.2118/15493-MS. ↩
-
F.I. Stalkup. Miscible disphcement. 1984. ↩
-
F. M. Orr, R. T. Johns, and B. Dindoruk. Development of miscibility in four-component vaporizing gas drives. In Proceedings of the 1991 SPE Annual Technical Conference and Exhibition, "449–460". 1991. ↩↩
-
R.T. Johns, F.J. Fayers, and Jr. Orr, F.M. Effect of gas enrichment and dispersion on nearly miscible displacements in condensing/vaporizing drives. SPE Advanced Technology Series, 2:paper SPE–24938–PA, 1994. doi:10.2118/24938-PA. ↩↩
-
C. L. Hearn and C. H. Whitson. Evaluating miscible and immiscible gas injection in the safah field, oman. In SPE Reservoir Simulation Symposium, paper SPE–29115–MS. Society of Petroleum Engineers, 1995. doi:10.2118/29115-MS. ↩
-
K. Ahmadi and R. T. Johns. Multiple-mixing-cell method for mmp calculations. SPE journal, 16:paper SPE–116823–PA, 2011. doi:10.2118/116823-PA. ↩
-
H. Zhao and Z. Fang. Improved multiple-mixing-cell method for accelerating minimum miscibility pressure calculations. SPE Journal, pages paper SPE–199360–PA, 2019. doi:10.2118/199360-PA. ↩↩
-
Jr. Orr, F.M., R.T. Johns, and B. Dindoruk. Development of miscibility in four-component co2 floods. SPE reservoir engineering, 8:paper SPE–22637–PA, 1993. doi:10.2118/22637-PA. ↩
-
Y. Wang and F. M. Orr. Calculation of minimum miscibility pressure. Journal of petroleum science and engineering, 27:paper SPE–39683–MS, 2000. doi:10.2118/39683-MS. ↩
-
L. Yarborough and L.R. Smith. Solvent and driving gas compositions for miscible slug displacement. Society of Petroleum Engineers Journal, 10:paper SPE–2543–PA, 1970. doi:10.2118/2543-PA. ↩
-
W.F. Yellig and R.S. Metcalfe. Determination and prediction of co2 minimum miscibility pressures. Journal of Petroleum Technology, 32:paper SPE–7477–PA, 1980. doi:10.2118/7477-PA. ↩
-
R. L. Christiansen and H. K. Haines. Rapid measurement of minimum miscibility pressure with the rising-bubble apparatus. SPE Reservoir Engineering, 2:paper SPE–13114–PA, 1987. doi:10.2118/13114-PA. ↩
-
K. Jessen and F. M. Orr. On interfacial-tension measurements to estimate minimum miscibility pressures. SPE reservoir evaluation & engineering, 11:paper SPE–110725–PA, 2008. doi:10.2118/110725-PA. ↩
-
D. N. Rao. A new technique of vanishing interfacial tension for miscibility determination. Fluid phase equilibria, 139:311–324, 1997. doi:https://doi.org/10.1016/S0378-381200180-5. ↩